Quote:
Originally Posted by Mungo
I have nitrogen and won't go back to air for several reasons
1. Does not expand or contract as much as air.
|
Yes it does. They want you to believe that Nitrogen is somehow more "pressure-stable" (for lack of better term). The nitrogen itself has NOTHING to do with it. The small amount of water vapor in the air is what contracts/expands more with changes in temperature. If you use a dryer with your compressor you'll get the same effect, since Nitrogen is usually free of water vapor. Also, the relatively small temperature changes your tires see will not show a significant difference between Nitrogen and standard compressed air.
Quote:
|
2. Nitrogen molecules are bigger than air so you will not lose pressure as fast.
|
Ok, this is a little bit believable. Nitrogen and Oxygen are both diatomic molecules (2 atoms per molecule), and even though the atomic size of oxygen is a little bigger, nitrogen his held together with a triple bond, effectively holding the atoms closer together. This source,
http://www.getnitrogen.org/pdf/graham.pdf claims that O2 permeates through rubber 3-4 times faster than Nitrogen. Ok, well there's still ~80% Nitrogen in the air, so less than 1/5th (20%) of the total volume of the air in your tires is leaking out that fast. The other molecules (CO2, water vapor, radon, etc) are much larger than Nitrogen, and will leak out even slower. So basically you have to ask yourself is that $40 worth only having to check your tire pressure every 2 months instead of 1 month and 3 weeks. Even though you should be checking at every fill up.
Quote:
|
3. I and my wife have noticed a smoother ride since switching to nitrogen.
|
As someone else stated here, it's called placebo effect. You may state that Nitrogen is lighter than air, so that's what makes the difference. Not really. 1 mole of Nitrogen molecules (6.023x10^23) weighs 28 grams. The same number of Oxygen molecules weighs 32 grams. At STP (Standard Temperature and Pressure) 1 mole of a gas will occupy 22.4L of volume. I don't know how much volume it takes to fill one of your tires, but let's say it's 44.8L just to keep this simple. Using the ideal gas law that tradosaurus listed below, you'll see that the weight of the air vs the weight of the Nitrogen in your tires will be a difference measured in grams. Not enough to make a difference in ANYTHING.
Quote:
|
4. Nitrogen contains no moisture so the inside of the tire won't rot like they do with air.
|
I think PQ was the one that said your tires won't be on your car long enough to rot. Besides, there's water vapor on the OUTSIDE of your tires, and I don't see many people rolling around with their tires rotting off from the outside. The only difference this might make is if you have old, unpainted steel wheels on your car it could cause them to corrode faster. Even then, they're not going to turn to dust or anything.
Quote:
|
I paid $49.00 for four tires at the dealer and when ever I need a top off at oil change time he does it for free. And by the way nitrogen is lighter than air and my gas milage went up by 4 MPG. Ask a reputable tire shop for their opinion. These are my reasons.
|
You said on a long road trip the only difference was the nitrogen in your tires and you MPG went up. Surely there were more variables than just that. If not done in a controlled environment, those conclusions are pretty much null and void.
Quote:
Originally Posted by tradosaurus
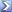
Gas law, PV = nRT for you nerds out there.
|
I'm glad someone else remembers this. This equation shows you that pressure change is COMPLETELY INDIFFERENT to the type of gas being used as temperature increases/decreases.
Verdict: Nitrogen is a waste of $40